Gene therapy in haemophilia
The most advanced clinical trials for gene therapy in haemophilia are using a particular type of carrier, called a vector, to deliver the gene therapy via the bloodstream to liver cells in the body. One important type is known as the AAV (adeno-associated virus) vector.
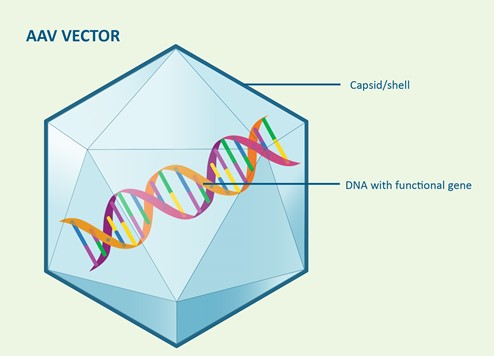
The aim is to deliver a therapeutic or functional version of the factor VIII or IX gene into the body, so that it then gives the right target cells in the body directions to produce factor VIII or IX that works properly.
DNA cannot be delivered orally, for example, in a tablet or syrup. When it is delivered through an injection or drip into a vein, the DNA needs to be protected or it will be destroyed in the bloodstream.
Some viruses are often used as vectors in gene therapy because they can deliver the new DNA by entering cells. They are very efficient at this, which is why so many viruses cause infections in us. Scientists have tested several viruses and removed any virus genes to insert the factor VIII or factor IX genes. The adeno-associated virus (AAV) has proven to be very effective in delivering new factor VIII or factor IX genes to animals and humans.
The viruses have a shell or capsid which protects the DNA and also targets particular body tissues. Factor VIII and IX are produced in the liver, so the AAV vector used in gene therapy for haemophilia is designed to target liver cells.
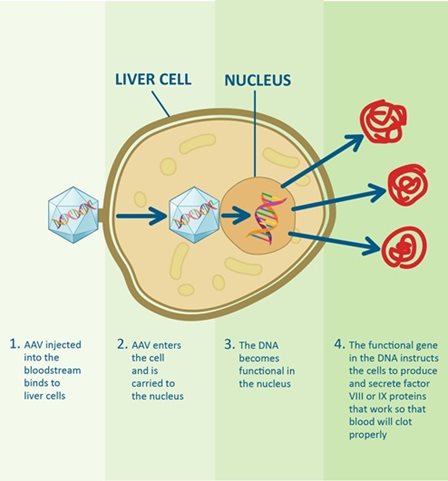
How gene therapy works in the body
1. AAV injected into the bloodstream binds to liver cells
2. AAV enters the cell and is carried to the cell nucleus
3. The DNA becomes functional in the nucleus
4. The functional gene in the DNA instructs the cells to produce and secrete factor VIII or IX proteins that work so blood will clot properly
Will using the AAV vector give me a disease?
The virus (AAV) does not cause illness in humans. The virus is also modified in the laboratory to replace the AAV DNA with the DNA containing the therapeutic gene. This means the AAV vector is very unlikely to cause disease when used in people, but instead will help to produce working factor VIII or factor IX.
