SUMIT PARIKH
Dr Sumit Parikh is the AHCDO ABDR Senior Research Fellow

Read the comment from HFA – The ongoing hepatitis C campaign
Routine screening of blood donations for the presence of hepatitis C virus (HCV) commenced in Australia in 1990. Super-heat treatment and other HCV viral inactivation manufacturing processes were also introduced for plasma-derived clotting factor concentrates used to treat bleeding disorders, and HCV-inactivated factor VIII (8) concentrate became available in Australia in 1990 and HCV-inactivated Prothrombinex® concentrate for factor IX (9) deficiency became available in 1993. Many patients with bleeding disorders in Australia were exposed to plasma-derived clotting factor concentrate contaminated with HCV prior to that time. (1) These patients have been living with HCV for more than 25 years and it has become a leading cause of morbidity and mortality in this group.(2)
Epidemiological studies suggest that living with HCV without achieving a Sustained Virological Response (SVR) to treatment is a higher risk for advanced liver disease, and that disease progression accelerates the longer the patient has been infected and as they age.(3) The current incidence and prevalence of HCV among patients with bleeding disorders in Australia is unknown, including treatment uptake and outcome. This raises a grave concern about the number of patients who may be undiagnosed with HCV, including those who are not undergoing any treatment or follow-up.
In recent times there have been significant improvements in medical technology to diagnose HCV and major advancements in hepatitis C treatment, including the availability of government-subsidised new direct acting antiviral (DAA) treatments, which have high cure rates and few if any side-effects. In 2017 Australian Haemophilia Centre Directors’ Organisation (AHCDO) conducted a nationwide study looking at the current health status of patients with HCV in the bleeding disorders population and evaluating the impact of subsidised DAA HCV medications on treatment uptake and outcome. Data was drawn from the Australian Bleeding Disorders Registry (ABDR), which was established in 1988, and includes some people who are now deceased. The results of this study were presented at the World Federation of Haemophilia (WFH) 2018 World Congress in Glasgow, UK.(4)
In summary, a total of 2166 patients with bleeding disorders in the ABDR who received plasma products prior to 1993 have been tested for HCV, with 894 patients HCV antibody positive (i.e., showing evidence of exposure to HCV). The majority of patients who are HCV antibody positive have haemophilia A (68%), haemophilia B (18%) or von Willebrand disease (11%). Almost half of the patients who are currently HCV antibody positive belong to the 41–60 years age group.
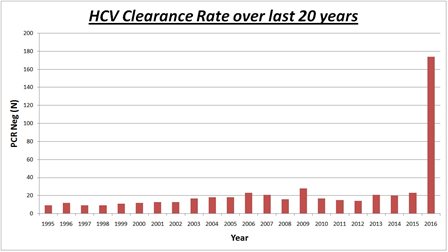
By 2017 around 60% of these patients had cleared the virus with or without treatment and are now PCR negative (i.e., show no evidence of ongoing infection with HCV in their blood), including a significant number of patients with mild or moderate bleeding disorders. Almost one-third of the patients who had cleared the virus had received subsidised DAA treatment in 2016, compared to the remainder who were treated with interferon-based therapy in the 18 years prior. This demonstrates a major advancement in the effectiveness and uptake of HCV treatment. A substantial number of remaining patients with HCV infection were undergoing treatment in 2017-18 which is a clear indication of the tremendous impact that the latest direct antiviral treatment have on the uptake and clearance rate of HCV.
A considerable number of patients who have received blood and/or blood products prior to 1993, however, have not yet been tested for HCV. The ongoing challenge is to identify all potential patients at risk of HCV infection and maximising this opportunity to eradicate HCV.
REFERENCES
1. Australia. Senate Community Affairs References Committee. Hepatitis C and the blood supply in Australia. Canberra: Senate Community Affairs References Committee Secretariat, 2004.
2. Northcott MJ, Ong WL, Walsh M, et al. Prevalence of transfusion-acquired hepatitis C in an Australian bleeding disorders population. Haemophilia 2013;19(6):847-52. <>
3. Poynard, T et al. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J of Hepatology 2001; 34:730-9.;
Walsh CE, Workowski K, Terrault NE, et al. Ledipasvir–sofosbuvir and sofosbuvir plus ribavirin in patients with chronic hepatitis C and bleeding disorders. Haemophilia 2017;23:198-206.
4. Parikh S, Tran H, McRae S. Uptake of subsidised hepatitis C direct acting antiviral treatment among patients with bleeding disorders in Australia [abstract]. Haemophilia 2018;24(Suppl. 5):1-218.
Haemophilia Foundation Australia acknowledges the Traditional Owners and Custodians of Country throughout Australia, the land, waters and community where we walk, live, meet and work. We pay our respects to Elders past and present and extend that respect to all Aboriginal and Torres Strait Islander peoples.
Sign up for the latest news, events and our free National Haemophilia magazine